Why Deep-tech companies need Product Managers
The commercialisation valley of death is due to lack of experienced operators
In the world of deep-tech, particularly in biotech and medtech, groundbreaking research often faces a steep challenge when it comes to commercialisation. This challenge, known as the "commercialisation valley of death," is where many promising innovations stall.
This is where you may expect me to post a graph of the valley of death, but let's skip the cliché. Anyone who’s spent time in a lab coat is well aware of it. Instead, let’s focus on how to actually fix this problem. My career, as a research scientist and as a tech product manager, has shown me that the missing link often lies in a lack of experienced operators who understand not just the science but the processes needed to bring products to market.
This is especially true in markets like Australia, where the biotech and medtech ecosystem isn’t as mature as in the US. The scarcity of successful deep-tech companies here means there’s a corresponding lack of experienced operators who’ve taken an idea from the lab all the way to the market.
This was reflected in an AusMedTech plenary session that I attended in 20221
Here is a write up from that Conference by Minter Ellison:
It has for some time been a constant challenge for MedTech companies in Australia to source and retain the right people and in the right numbers. . .
In relative world terms, it is said that Australia has a small pool of qualified medical device people. This reinforces the need to be constantly engaging with prospective and existing staff and ensuring that they are motivated and incentivised to remain in the workforce.
How principles used by tech Product Managers could help
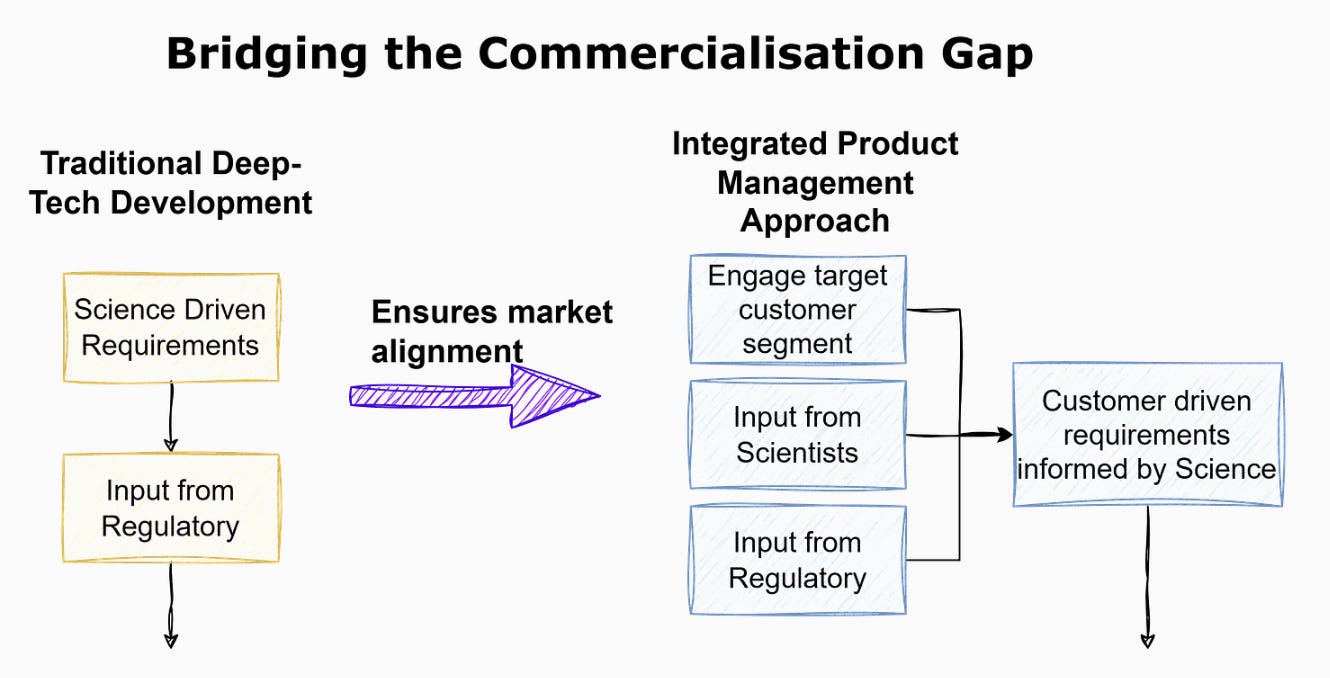
Tech product managers bring proven and high-impact processes that can be game-changers for deep-tech companies. Agile development, user-centered design, and cross-functional team collaboration are just a few examples of the methodologies that can transform how deep-tech innovations move from concept to market. In software, these approaches have led to rapid product cycles, continuous improvement, and a strong alignment between product development and market needs.
Deep-tech companies, on the other hand, often struggle with long development cycles, regulatory hurdles, and a focus that can be too inward-looking. Having worked in regulated environments, including Next-Gen Sequencing Pathology companies (ISO 15189) and Software as a Medical Device (SaMD) (ISO 13485), I’ve seen firsthand that even traditional university spinout deep-tech companies can benefit from practices used in the tech industry to better deliver essential products and services to their customers.
It's a misconception that regulatory ISO standards require a waterfall development approach, where long development lead times burn capital and create a high-risk gamble for a successful product launch. In fact, frameworks like TIR-45 provide guidance on integrating agile practices within regulated environments for SaMDs.
By incorporating compliance activities into each sprint—such as documentation, verification, and validation—deep-tech companies can ensure that regulatory requirements are met incrementally. This approach reduces the risk of non-compliance surprises late in the development cycle and accelerates time-to-market. Regulatory bodies are primarily concerned with the quality and safety of the final product, not the specific development methodology used to achieve it.
Even with physical products such as lab-based tests or Research Use Only (RUO) test kits, value can still be expertly planned and delivered in strategic milestones that move the needle on a company’s OKRs.
By adopting software PM practices, deep-tech firms can not only accelerate their development timelines but also ensure that their innovations are aligned with market demands and regulatory requirements from the outset. This integration is crucial for avoiding the pitfalls that lead to the commercialisation valley of death.
Building a Community of Experienced Deep-tech Operators
EmbarkBio is a hub for professionals who are ready to bridge the gap between science and market. This is a space where we’ll explore how deep-tech companies can adopt and adapt the best practices from product management to drive their innovations forward.
What to Expect
Here’s what you can expect from EmbarkBio:
Fortnightly Posts & Monthly Youtube Videos: To begin, I’ll aim to post twice a month with detailed explorations of how product management methodologies and their applications to deep-tech industries, with a focus on product management, agile development, and market alignment. I’ll also be posting monthly deep dive videos on my YouTube channel that I will link through to this site with supplementary information.
Content for Executives, Managers and Specialists in Deep-tech companies: This content is primarily written based on my experience in biotech and medtech and aimed towards those who have mid to senior level positions within these types companies. The insights here likely also apply to other deep-tech and regulated industries and may be of relevance to you.
Join the Journey
This is just the beginning. I invite you to subscribe to EmbarkBio and be part of a community that’s dedicated to transforming deep-tech challenges into opportunities for growth and innovation. Together, we’ll explore how to apply the strategic processes from software tech to drive success in the biotech and medtech industries.
Incidentally I was also invited to speak at a different panel at this same event, about “The diagnostics landscape post COVID-19 - Keeping up with the changing pace”. A topic that I can dive into deeper another time.

